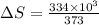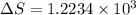Physics, 09.12.2019 20:31 cassiemyers60
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to steam at 100 degree c. assume that the heat of vaporization of water is 2256 times 103 j/kg. calculate the change in entropy when 1.00 kg of ice is melted at 0 degree c. assume that the heat of fusion of water is lf = 3.34 times 105j/kg. is the change entropy greater for melting or for vaporization? the change entropy greater for melting the change entropy greater for vaporization

Answers: 3


Another question on Physics

Physics, 21.06.2019 13:30
Would corn syrup, molasses, or pancake syrup make a good lubricant in a car engine? explain your answer.
Answers: 1

Physics, 22.06.2019 13:10
Which additional product balances the reaction h2so4 + 2naoh → na2so4 + 2h2o 2oh h2o2 h3o
Answers: 1

Physics, 22.06.2019 19:00
Friction removes energy from objects in motion. which statement best describes how this works? a) friction transforms ke into thermal energy b) friction transfers thermal energy to ke c) friction transforms te into pe d) friction transforms pe into ke e) friction transfers ke into pe
Answers: 1

Physics, 22.06.2019 19:30
Aplayground slide is 8.80 ft long and makes an angle of 25.0° with the horizontal. a 63.0-kg child, initially at the top, slides all the way down to the bottom of the slide. (a) choosing the bottom of the slide as the reference configuration, what is the system's potential energy when the child is at the top and at the bottom of the slide? what is the change in potential energy as the child slides from the top to the bottom of the slide? (include the sign of the value in your answer.)
Answers: 3
You know the right answer?
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to...
Questions


Social Studies, 29.07.2021 02:40



Mathematics, 29.07.2021 02:40





Mathematics, 29.07.2021 02:40



Mathematics, 29.07.2021 02:40


English, 29.07.2021 02:40

Mathematics, 29.07.2021 02:40

Mathematics, 29.07.2021 02:40

Advanced Placement (AP), 29.07.2021 02:40


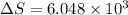
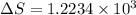
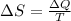
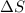 is change of entropy,
is change of entropy, 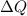 is change of heat and T is absolute temperature in kelvin
is change of heat and T is absolute temperature in kelvin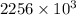 J/kg.
J/kg.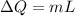
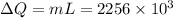
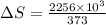
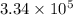 J/kg.
J/kg.![3.34 \times 10^{5}=334 \times 10^{3} J/kg.[tex]\Delta Q = mL = 334 \times 10^{3}](/tpl/images/0410/3794/17bda.png)