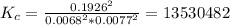Physics, 10.12.2019 02:31 mooncake9090
Areaction mixture in a 3.67 l flask at a certain temperature initially contains 0.763 g h2 and 96.9 g i2, at equilibrium, the flask contains 90.4 g hi. calculate the equilibrium constant (kc) for the reaction at this temperature.

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:00
Which safety measures should you follow during a thunderstorm? check all that apply. (a) avoid touching anything that conducts electricity. (b) avoid touching a person who has been struck by lightning. (c) go outside. (d) keep your computer turned off. (e) stay out of water. (f) stay inside.
Answers: 2

Physics, 22.06.2019 20:30
Ascientist notices that an oil slick floating on water when viewed from above has many different colors reflecting off the surface, making it look rainbow-like (an effect known as iridescence). she aims a spectrometer at a particular spot and measures the wavelength to be 750 nm (in air). the index of refraction of water is 1.3
Answers: 1

Physics, 23.06.2019 03:00
Ablock of mass m slides on a horizontal frictionless table with an initial speed v0 . it then compresses a spring of force constant k and is brought to rest. the acceleration of gravity is 9.8 m/s2 . how much is the spring compressed x from its natural length?
Answers: 3

Physics, 23.06.2019 10:50
The is a pea sized structure in the brain that is involved in many aspects of motivation, including sex, aggression,and hunger
Answers: 1
You know the right answer?
Areaction mixture in a 3.67 l flask at a certain temperature initially contains 0.763 g h2 and 96.9...
Questions




Mathematics, 21.04.2021 23:50


History, 21.04.2021 23:50


English, 21.04.2021 23:50

History, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50

Geography, 21.04.2021 23:50


Mathematics, 21.04.2021 23:50





Biology, 21.04.2021 23:50


Business, 21.04.2021 23:50