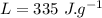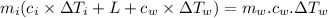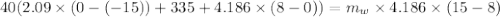A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:30
What is the relationship between the wavelength of a wave and it's energy?
Answers: 1


Physics, 22.06.2019 11:00
Alarge box of mass m is pulled across a horizontal frictionless surface by a horizontal rope with tension t. a small box of mass m sits on top of the large box. the coefficients of static and
Answers: 1

Physics, 23.06.2019 00:30
Language is not the only way that humans communicate. describe how we use sound, touch, chemicals, and sight to communicate. need answer asap
Answers: 2
You know the right answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions



Mathematics, 05.08.2020 14:01



Mathematics, 05.08.2020 14:01





Mathematics, 05.08.2020 14:01

Mathematics, 05.08.2020 14:01

Mathematics, 05.08.2020 14:01


Mathematics, 05.08.2020 14:01


History, 05.08.2020 14:01

World Languages, 05.08.2020 14:01


Mathematics, 05.08.2020 14:01

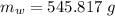
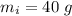 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture, 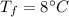 specific heat of ice,
specific heat of ice, 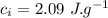 specific heat of water,
specific heat of water, 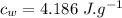 Latent heat of fusion of water,
Latent heat of fusion of water,