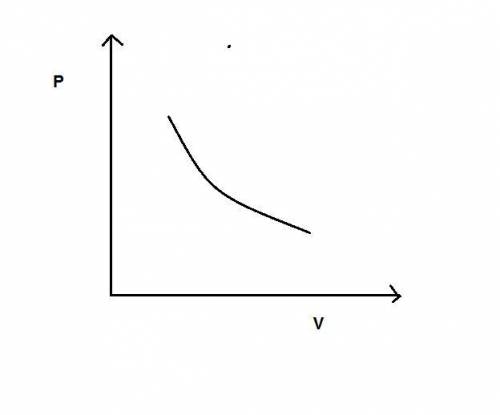
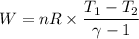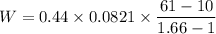During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0 ∘c. the argon may be treated as an ideal gas.(a) draw a pv-diagram for this process.
(b) how much work does the gas do?
(c) what is the change in internal energy of the gas? explain.

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:30
Not all tiles will be usedidentify the element present in each star based on the gaps observed in wavelengths of its light refer to this table containing absorption wavelengths (in nm) of several different elements the table ishelium 447, 502 ,587 ,668carbon 427 ,515, 600 ,678sulfur 425, 565, 639, 675xenon 484, 590, 687calcium 429 ,527, 593, 645silver 421 ,521, 662
Answers: 3

Physics, 22.06.2019 07:00
Within a pendulum, as potential energy decreases, energy increases. a. heat b. kinetic c. frictional d. gravitational
Answers: 1

Physics, 22.06.2019 14:20
How many atoms of nitrogen are in the chemical formula ni(w on
Answers: 1

Physics, 22.06.2019 20:00
Awave has a wavelength of 7 mm and a frequency of 19 hertz. what is its speed?
Answers: 1
You know the right answer?
During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0...
Questions




Social Studies, 06.08.2019 23:30











Computers and Technology, 06.08.2019 23:30