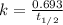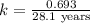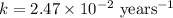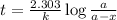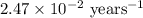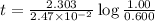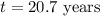Physics, 12.12.2019 04:31 erik1franks
Strontium−90 is one of the products of the fission of uranium−235. this strontium isotope is radioactive, with a half-life of 28.1 yr. calculate how long (in yr) it will take for 1.00 g of the isotope to be reduced to 0.600 g by decay.

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:40
In physics the desire of an object to keep doing what it is doing is termed?
Answers: 1

Physics, 21.06.2019 23:30
After a big snowfall, you take your favorite rocket-powered sled out to a wide field. the field is 195 m across, and you know that your sled accelerates at a rate of 3.65 m/s2 when the rocket is on. how much time will it take the sled to cross the field starting from rest, assuming the rocket is on the whole time?
Answers: 1

Physics, 22.06.2019 03:00
Which law represents the thermodynamic statement of the conservation of energy of a system? a. the fourth law b. the first law c. the second law d. the third law
Answers: 2

You know the right answer?
Strontium−90 is one of the products of the fission of uranium−235. this strontium isotope is radioac...
Questions

History, 03.12.2020 07:10


Biology, 03.12.2020 07:10

Geography, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10



Physics, 03.12.2020 07:10

English, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

Biology, 03.12.2020 07:10

Arts, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

English, 03.12.2020 07:10