
Physics, 13.12.2019 04:31 kmoo176394
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3 is compressed adiabatically to a volume of 0.0400 m^3.
(a) what is the final pressure?
(b) how much work is done by the gas?
(c) what is the ratio of the final temperature of the gas to its initial temperature? is the gas heated or cooled by this compression?

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:20
Very large accelerations can injure the body, especially if they last for a considerable length of time. one model used to gauge the likelihood of injury is the severity index ( ), defined as =/ . in the expression, is the duration of the accleration, but is not equal to the acceleration. rather, is a dimensionless constant that = the number of multiples of that the acceleration is equal to.in one set of studies of rear-end collisions, a person's velocity increases by 13.7 km/h with an acceleration of 36.0 m/s2 . let the + direction point in the direction the car is traveling. what is the severity index for the collision?
Answers: 1

Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 22.06.2019 20:00
Using the free-body diagram, calculate the net force acting on the sled. is the sled in a state of dynamic equilibrium?
Answers: 3

Physics, 22.06.2019 20:50
An ideal otto cycle has a compression ratio of 8. at the beginning of the compression process, air is at 95 kpa and 27°c, and 750 kj/kg of heat is transferred to air during the constant-volume heat-addition process. assuming constant specific heats at room temperature, determine (a) the pressure and temperature at the end of the heat-addition process, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. (4390 kpa, 1730 k; 423 kj/kg; 56.4%; 534 kpa)
Answers: 1
You know the right answer?
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3...
Questions

Geography, 14.09.2020 19:01

French, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Social Studies, 14.09.2020 19:01

Biology, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

French, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

French, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

English, 14.09.2020 19:01

French, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

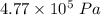 .
.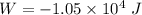 .
.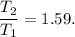 and gas is heated.
and gas is heated.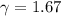 .
.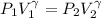 .
.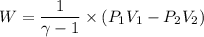 .
.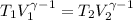 .
.

