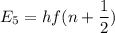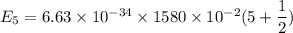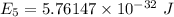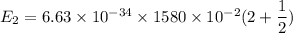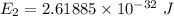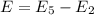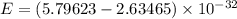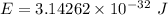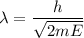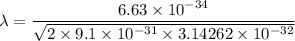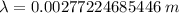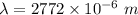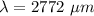Physics, 14.12.2019 01:31 hemolelekeakua
It is common for atomic physicists to quote a frequency in cm^−1, since f=c/λ=3× 10^10 cm/s/λ. the vibrational frequency for the interatomic distance in o2 is f=1580 cm^−1. what wavelength of light in micrometers is emitted when an o2 molecule jumps from the vibrational level (for which en=hf(n+1/2)) with n=5 to n=2?

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 01:30
The passing of heat through a material while the material itself stays in place. a. radiation b. conduction c. convection
Answers: 2

Physics, 22.06.2019 08:30
Hey student studies gravity using objects that have the same mass which two objects have the greatest gravitational force acting between them a. 100kg 1.0m 100kg b. 100kg 2.0m 100kg c. 100kg 2.0m 100kg big d. 100kg big 3.0m 100kg big
Answers: 1

Physics, 22.06.2019 11:40
Consider the following position function. find (a) the velocity and the speed of the object and (b) the acceleration of the object. bold r left parenthesis t right parenthesisr(t)equals=left angle 6 t superscript 4 baseline comma 2 t cubed right angle6t4,2t3 for tgreater than or equals≥0
Answers: 3
You know the right answer?
It is common for atomic physicists to quote a frequency in cm^−1, since f=c/λ=3× 10^10 cm/s/λ. the v...
Questions






Mathematics, 09.01.2021 04:10

English, 09.01.2021 04:10

Computers and Technology, 09.01.2021 04:10

English, 09.01.2021 04:20



Biology, 09.01.2021 04:20

Mathematics, 09.01.2021 04:20





Advanced Placement (AP), 09.01.2021 04:20