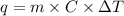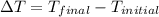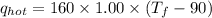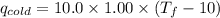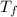Find the final equilibrium temperature when 10.0 g of milk at 10.0°c is added to 160 g of coffee at 90.0°c.
(assume the specific heats of coffee and milk are the same as water and neglect the heat capacity of the container.) cwater = 1.00 cal/g·°c = 4186 j/kg·°c
a. 85.3°c
b. 77.7°c
c. 71.4°c
d. 66.7°c

Answers: 3


Another question on Physics

Physics, 22.06.2019 08:30
Abike rider starts from rest and accelerates 28.0 meters down a slope in 5.00 seconds. what is her acceleration? select one: a. 3.21 m/sec2 b. 1.75 m/sec2 c. 9.80 m/sec2 d. 2.24 m/sec2
Answers: 3

Physics, 22.06.2019 17:00
If you wanted to move an electron from the positive to the negative terminal of the battery, how much work w would you need to do on the electron? enter your answer numerically in joules.
Answers: 1


Physics, 22.06.2019 18:00
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3
You know the right answer?
Find the final equilibrium temperature when 10.0 g of milk at 10.0°c is added to 160 g of coffee at...
Questions



Mathematics, 23.04.2020 20:39

Mathematics, 23.04.2020 20:39


History, 23.04.2020 20:39


History, 23.04.2020 20:39

Social Studies, 23.04.2020 20:39






Mathematics, 23.04.2020 20:39

Mathematics, 23.04.2020 20:39


Mathematics, 23.04.2020 20:40


Biology, 23.04.2020 20:40