
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. sufficient pcl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250 °c. calculate the pressure of pcl5 after the system has reached equilibrium.
a. 1.50 atm
b. 1.24 atm
c. 4.24 atm
d. 0.94 atm
e. 1.12 atm

Answers: 3


Another question on Physics


Physics, 22.06.2019 10:30
Light from a sodium lamp passes through a diffraction grating that has 1000 slits per millimeter. the interference pattern is viewed on a screen 1.000 m behind the grating. the first (m = 1) two bright yellow fringes that are visible are 0.7288 m and 0.7300 m from the central maximum. what are the wavelengths of these two fringes?
Answers: 2


Physics, 22.06.2019 14:30
Slab pull” is a type of tectonic plate movement that occurs due to the forces of mantle convection and results in the subduction of the lithosphere true or false
Answers: 2
You know the right answer?
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. suffici...
Questions

Mathematics, 21.08.2019 23:30





Mathematics, 21.08.2019 23:30

Mathematics, 21.08.2019 23:30


History, 21.08.2019 23:30

Chemistry, 21.08.2019 23:30



Mathematics, 21.08.2019 23:30


English, 21.08.2019 23:30




Mathematics, 21.08.2019 23:30

Social Studies, 21.08.2019 23:30

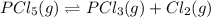
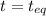 2.74-x x x
2.74-x x x
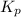 for the given reaction follows:
for the given reaction follows:
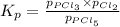
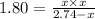
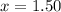
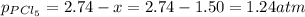
 at equilibrium is 1.24 atm
at equilibrium is 1.24 atm

