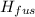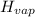Physics, 17.12.2019 01:31 Paulalex8765
How much heat energy is required to vaporize a 1.0-g ice cube at 0°c? the heat of fusion of ice is 80 cal/g.
the heat of vaporization of water is 540 cal/g, and cwater = 1.00 cal/g⋅°c.
a. 620 cal
b. 720 cal
c. 820 cal
d. 1 kcal

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:00
This question is worth 54 points ! rock falls from a ledge to the ground what energy conversion occurs during this process a potential to kinetic b kinetic to potential c thermal to mechanical d chemical to mechanical
Answers: 2

Physics, 22.06.2019 17:00
The force it would take to accelerate an 900-kg car at the rate of 6m/s2
Answers: 1

Physics, 22.06.2019 20:20
The temperature in gavin's oven is a sinusoidal function of time. gavin sets his oven so that it has a maximum temperature of 280°f and a minimum temperature of 240°. once the temperature hits 280°, it takes 20 minutes before it is 280° again. gavin's cake needs to be in the oven for 30 minutes at temperatures at or above 270°. he puts the cake into the oven when it is at 260° and rising. how long will gavin need to leave the cake in the oven? (round your answer to the nearest minute.)
Answers: 3

Physics, 23.06.2019 01:30
Relative to the ground below, how many joules of potential energy does a 1000-kg boulder have at the top of a 5-m ledge?
Answers: 1
You know the right answer?
How much heat energy is required to vaporize a 1.0-g ice cube at 0°c? the heat of fusion of ice is...
Questions

Chemistry, 04.12.2020 02:30



Biology, 04.12.2020 02:30

Mathematics, 04.12.2020 02:30


Mathematics, 04.12.2020 02:30




Mathematics, 04.12.2020 02:30

Mathematics, 04.12.2020 02:30

Biology, 04.12.2020 02:30

English, 04.12.2020 02:30

Mathematics, 04.12.2020 02:30

Biology, 04.12.2020 02:30



Mathematics, 04.12.2020 02:30