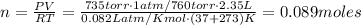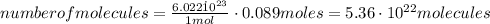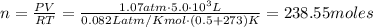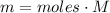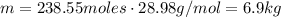Calculate the number of molecules in a deep breath of air whose volume is 2.35 l at body temperature, 37 ∘c, and a pressure of 735 torr. express the answer in molecules to three significant figures. nn = nothing molecules request answer part b the adult blue whale has a lung capacity of 5.0×103 l. calculate the mass of air (assume an average molar mass 28.98 g/mol) contained in an adult blue whale’s lungs at 0.5 ∘c and 1.07 atm, assuming the air behaves ideally. express the answer in kilograms to two significant figures. mm = nothing kg

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:30
Aforce of 9.00 newtons acts at an angle of 19.0 to the horizontal. what is its component in the horizontal direction?
Answers: 2

Physics, 22.06.2019 03:10
Aphysical change is a change in the size, shape,, or stafe of matter true or false
Answers: 1

Physics, 22.06.2019 07:40
Astudent creates a model of a closed ecosystem by filling a glass tank half full with water, then adding 10 snails and two small aquatic plants. the next day, all the snails are dead. what is the most likely cause of their death?
Answers: 3

Physics, 22.06.2019 10:30
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2
You know the right answer?
Calculate the number of molecules in a deep breath of air whose volume is 2.35 l at body temperature...
Questions

Social Studies, 09.03.2021 20:10


Mathematics, 09.03.2021 20:10


History, 09.03.2021 20:10


Mathematics, 09.03.2021 20:10

English, 09.03.2021 20:10



Social Studies, 09.03.2021 20:10






Mathematics, 09.03.2021 20:10

Mathematics, 09.03.2021 20:10


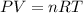 (1)
(1)