
Physics, 17.12.2019 17:31 ysabel0420
10. a chemical reaction was run in 215.5 ml of h2o. during the reaction, the temperature of the h2o changes from 34.4 oc to 57.6 oc. how much heat (in kj) did the chemical reaction produce? for h2o, cp= 4.184 j/(g · oc). assume the mass of the solution is 215.5 g. enter your answer as a positive decimal number.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:00
The image below shows numerous volcanic mountains in the pacific northwest. what is the most likely cause of the volcanic and earthquake activity in this region?
Answers: 2

Physics, 22.06.2019 12:40
Find the equation for the plane through upper p 0 left parenthesis negative 4 comma negative 8 comma negative 5 right parenthesis perpendicular to the following line. xequalsnegative 4 minus t, yequalsnegative 8 plus 2 t, zequals3 t, minusinfinityless thantless thaninfinity
Answers: 2


Physics, 23.06.2019 06:00
If we decrease the amount of force and keep all other factors the same what will happen to the amount of work
Answers: 1
You know the right answer?
10. a chemical reaction was run in 215.5 ml of h2o. during the reaction, the temperature of the h2o...
Questions


Mathematics, 25.03.2020 04:09

Mathematics, 25.03.2020 04:09

Mathematics, 25.03.2020 04:09

History, 25.03.2020 04:09

Mathematics, 25.03.2020 04:09

Mathematics, 25.03.2020 04:09

Biology, 25.03.2020 04:09



Mathematics, 25.03.2020 04:09





Mathematics, 25.03.2020 04:10


Mathematics, 25.03.2020 04:10


Mathematics, 25.03.2020 04:10

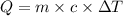
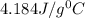
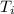 = 34.4°C
= 34.4°C
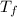 = 57.6°C
= 57.6°C
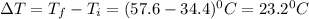
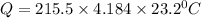
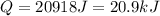 (1kJ=1000J)
(1kJ=1000J)

