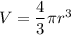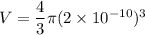Physics, 18.12.2019 04:31 silviamgarcia
The size (radius) of an oxygen molecule is about 2.0 ×10−10 m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
express your answer using one significant figure.
p = ? pa

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:30
11. you want to calculate the displacement of an object thrown over a bridge. using -10 m/s2 for acceleration due to gravity, what would be the total displacement of the object if it took 8 seconds before hitting the water?
Answers: 1


Physics, 22.06.2019 18:10
A200-n force is applied to the foot-operated air pump. the return spring s exerts a 2.6-n·m moment on member oba for this position. determine the corresponding compression force c in the cylinder bd. if the diameter of the piston in the cylinder is 40 mm, estimate the air pressure generated for these conditions. state any assumptions. enter a positive number for the compression force c.
Answers: 2

You know the right answer?
The size (radius) of an oxygen molecule is about 2.0 ×10−10 m. make a rough estimate of the pressure...
Questions

Mathematics, 15.10.2019 23:30






Mathematics, 15.10.2019 23:30



Geography, 15.10.2019 23:30

Chemistry, 15.10.2019 23:30


Mathematics, 15.10.2019 23:30




Mathematics, 15.10.2019 23:30

Mathematics, 15.10.2019 23:30

History, 15.10.2019 23:30