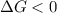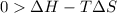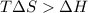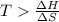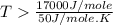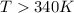One might be tempted to say that exothermic processes are always spontaneous since the system is emitting energy (heat) in order to reach a (preferred) lower energy state. however, as we have just investigated, the spontaneous process for polymers is endothermic. this reveals that we must consider entropy changes when determining the nature of spontaneity. the most probable configuration of a system and its surroundings, naturally, is the one that will be observed. the condition for spontaneity can be recast using the concept of the free energy of the system, where a change in free energy results both from changes in the enthalpy (which includes internal potential and kinetic energies) and the entropy (the number of states accessible to the system). δ g = δ h − t δ s.
an unknown chemical reaction undergoes an enthalpy change of δ h =17 kj/mol while the entropy increases by δ s =50 j/(mol * k).
above what temperature (in kelvin) does this reaction occur spontaneously?

Answers: 1


Another question on Physics

Physics, 21.06.2019 13:40
Which of the statements about waves is true? a. in longitudinal waves, particles move perpendicular to the direction the wave is traveling.b. both transverse and longitudinal waves can travel through water c. electromagnetic waves are a type of longitudinal waves.d. in a transverse wave, particles move parallel to the direction the wave is traveling.
Answers: 1

Physics, 21.06.2019 21:30
Aquantity of gas has a volume of 1.5 m3 and an absolute pressure of 95 kpa. when the gas is compressed to a volume of 0.5 m3, what is the new absolute pressure of the gas? (assume that there’s no change in temperature.)
Answers: 3

Physics, 22.06.2019 06:10
Which transition by an electron will release the greatest amount of energy? oa ob oc od
Answers: 2

Physics, 22.06.2019 16:30
In a hydrogen molecule there are a total of four charges, 2 protons in the two nuclei, and 2 electrons. how many unique charge-pairs are there (without double counting)?
Answers: 3
You know the right answer?
One might be tempted to say that exothermic processes are always spontaneous since the system is emi...
Questions


Mathematics, 03.12.2020 05:50

Mathematics, 03.12.2020 05:50

Computers and Technology, 03.12.2020 05:50


Computers and Technology, 03.12.2020 05:50

Law, 03.12.2020 05:50



Chemistry, 03.12.2020 05:50

Mathematics, 03.12.2020 05:50


French, 03.12.2020 05:50

History, 03.12.2020 05:50

English, 03.12.2020 05:50


Biology, 03.12.2020 05:50



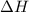 = 17 KJ/mole = 17000 J/mole
= 17 KJ/mole = 17000 J/mole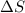 = 50 J/mole.K
= 50 J/mole.K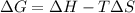
 is negative or we can say that the value of
is negative or we can say that the value of