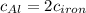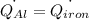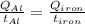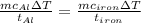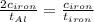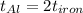Physics, 18.12.2019 22:31 Amaris0901
The specific heat capacity of aluminum is about twice that of iron. consider two blocks of equal mass, one made of aluminum and the other one made of iron, initially in thermal equilibrium.
heat is added to each block at the same constant rate until it reaches a temperature of 500 k. which of the following statements is true?
a. the iron takes less time than the aluminum to reach the final temperature.
b. the aluminum takes less time than the iron to reach the final temperature.
c. the two blocks take the same amount of time to reach the final temperature.

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
Individuals who live below the poverty line get seriously ill more often than those who do not what could be the hidden variable in this situation?
Answers: 3

Physics, 22.06.2019 10:20
Electromagnetic induction. a coil of wire contains n turns and has an electrical resistance r. the radius of each turn is a. initially, inside the coil there exists a uniform magnetic field of magnitude b0 parallel to the axis of the coil. the magnetic field is then reduced slowly. the current induced in the coil is i. how long does it take for the magnitude of the uniform field to drop to zero?
Answers: 1

Physics, 22.06.2019 14:20
The energy released by a nuclear fusion reaction is produced when
Answers: 1

Physics, 22.06.2019 19:30
Which type of energy would have nothing to do with ironing clothes? a. heat b. chemical c. electrical d. mechanical
Answers: 1
You know the right answer?
The specific heat capacity of aluminum is about twice that of iron. consider two blocks of equal mas...
Questions

English, 05.05.2020 06:27

Mathematics, 05.05.2020 06:27




Mathematics, 05.05.2020 06:27


Mathematics, 05.05.2020 06:27

Arts, 05.05.2020 06:27

Mathematics, 05.05.2020 06:27

Biology, 05.05.2020 06:27


Chemistry, 05.05.2020 06:27




Chemistry, 05.05.2020 06:27