
Physics, 19.12.2019 06:31 kaywendel2008
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with =n10 to an orbital with =n8. round your answer to 3 significant digits.

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:00
These models show the electron structures of two different nonmetal elements. which element is likely more reactive, and why? element 1 is more reactive because it has fewer electron shells and is toward the top of its group on the periodic table. element 1 is more reactive because it has more electrons in its valence shell and is farther to the right on the periodic table. element 2 is more reactive because it does not have a valence shell close to the nucleus, so it will attract electrons. element 2 is more reactive because it does not have a full valence shell, so it will attract electrons.
Answers: 2

Physics, 21.06.2019 18:00
Which surface feature of the moon is characterized by mountainous areas? terrae craters maria regolith
Answers: 1

Physics, 21.06.2019 19:00
An object is located 30.0 cm from a concave mirror. the focal length is 15.0 c,. what is the image distance? a. 30.0 cm b. -10 cm c. 10.0 cm d. -30.0 cm
Answers: 1

Physics, 22.06.2019 05:30
What is a neurotransmitter involved in mood reward, addiction, and motor behaviors?
Answers: 3
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in...
Questions

Chemistry, 25.09.2019 20:00

Advanced Placement (AP), 25.09.2019 20:00

History, 25.09.2019 20:00

History, 25.09.2019 20:00


Biology, 25.09.2019 20:00


English, 25.09.2019 20:00



English, 25.09.2019 20:00

English, 25.09.2019 20:00

English, 25.09.2019 20:00



English, 25.09.2019 20:00


English, 25.09.2019 20:00

English, 25.09.2019 20:00

Computers and Technology, 25.09.2019 20:00

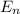 = - k²e² / 2m (1 / n²)
= - k²e² / 2m (1 / n²)
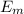 = - k²e² / 2m (1 /
= - k²e² / 2m (1 / 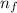 ² -1 /
² -1 / 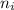 ²)
²)


