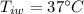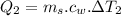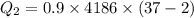Physics, 19.12.2019 23:31 leothedrifter
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. to see why, calculate the energy absorbed from a climber's body under the following conditions. the specific heat of ice is 2100 j/kg? c? , the latent heat of fusion is 333 kj/kg, the specific heat of water is4186 j/kg? c? .
a) calculate the energy absorbed from a climber's body if he eats 0.90
kg of -15? c snow which his body warms to body temperature of 37? c.
b) calculate the energy absorbed from a climber's body if he melts 0.90
kg of -15? c snow using a stove and drink the resulting 0.90kg of water at 2? c, which his body has to warm to 37? c.

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:30
The particle in a two-dimensional well is a useful model for the motion of electrons around the indole ring (3), the conjugated cycle found in the side chain of tryptophan. we may regard indole as a rectangle with sides of length 280 pm and 450 pm, with 10 electrons in the conjugated p system. as in case study 9.1, we assume that in the ground state of the molecule each quantized level is occupied by two electrons. (a) calculate the energy of an electron in the highest occupied level. (b) calculate the frequency of radiation that can induce a transition between the highest occupied and lowest unoccupied levels. 9.27 electrons around the porphine ring (4), the conjugated macrocycle that forms the structural basis of the heme group and the chlorophylls. we may treat the group as a circular ring of radius 440 pm, with 20 electrons in the conjugated system moving along the perimeter of the ring. as in exercise 9.26, assume that in the ground state of the molecule quantized each level is occupied by two electrons. (a) calculate the energy and angular momentum of an electron in the highest occupied level. (b) calculate the frequency of radiation that can induce a transition between the highest occupied and lowest unoccupied levels.
Answers: 1

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1

Physics, 22.06.2019 15:10
What does si stand for,when referring to a system of measurement
Answers: 1

You know the right answer?
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. to see why,...
Questions

English, 21.04.2020 18:55


Mathematics, 21.04.2020 18:55

Mathematics, 21.04.2020 18:55



Mathematics, 21.04.2020 18:55

Mathematics, 21.04.2020 18:55


Chemistry, 21.04.2020 18:55

English, 21.04.2020 18:55


Mathematics, 21.04.2020 18:55




Mathematics, 21.04.2020 18:55



Mathematics, 21.04.2020 18:55

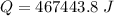
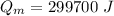
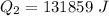
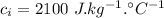 latent heat of fusion of ice,
latent heat of fusion of ice, 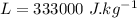 specific heat of water,
specific heat of water, 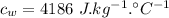
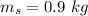 initial temperature of snow,
initial temperature of snow,  Final temperature of the consumed mass,
Final temperature of the consumed mass, 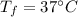
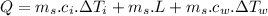
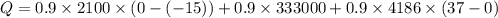
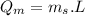
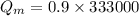
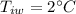 final temperature of water,
final temperature of water,