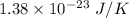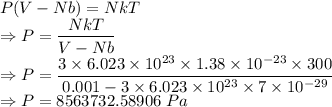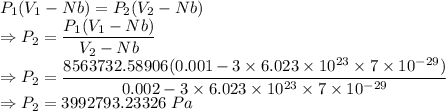Physics, 19.12.2019 23:31 bobtothemaxthe1st
Compressed gases aren't ideal. let's consider a gas that's non-ideal only because the volume available to each of the n molecules is reduced because each other molecule occupies volume v. instead of pv=nkt, we get: p(v-nb)=nkt. let b=7 × 10^-29 m3. let's look at 3 moles of this gas at t=300k starting in 0.001 m3 volume.
1) what's the initial value of the pressure? p_initial = ?
2) the gas expands isothermally to 0.002 m3. what's the final pressure? p_final = ?
3) how much work did the gas do in this isothermal expansion? w = ?

Answers: 1


Another question on Physics

Physics, 21.06.2019 14:50
Use the empirical rule. the mean speed of a sample of vehicles along a stretch of highway is 70 miles per hour, with a standard deviation of 4 miles per hour. estimate the percent of vehicles whose speeds are between 66 miles per hour and 74 miles per hour. (assume the data set has a bell-shaped distribution.)
Answers: 3

Physics, 21.06.2019 23:00
If an inclined plane is 5 m long and 2 m high, what is its mechanical advantage? a. 2.5 b. 3 c. 7 d. 10
Answers: 1

Physics, 22.06.2019 09:00
As a pendulum bob swings back and forth several times, the maximum height it reaches becomes less and less. this is because more and more of the pendulum bob's energy is being transformed into a. heat energy b. kinetic energy c. potential energy d. kinetic energy and potential energy
Answers: 2

Physics, 22.06.2019 11:00
Aperson walks first at a constant speed of 4.89 m/s along a straight line from point a to point b and then back along the line from b to a at a constant speed of 2.95 m/s. what is the average speed over the entire trip?
Answers: 1
You know the right answer?
Compressed gases aren't ideal. let's consider a gas that's non-ideal only because the volume availab...
Questions


English, 23.02.2021 18:10


English, 23.02.2021 18:10

History, 23.02.2021 18:10

Social Studies, 23.02.2021 18:10

History, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

History, 23.02.2021 18:10


Mathematics, 23.02.2021 18:10


Business, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10





Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

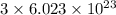
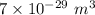
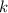 = Boltzmann constant =
= Boltzmann constant =