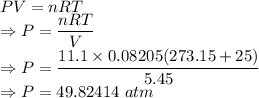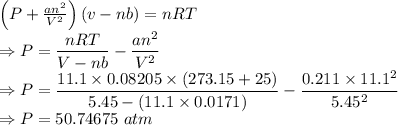Physics, 20.12.2019 01:31 moisealafleur
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the ideal gas equation and (b) the van der waals equation. (for neon, a = 0.211 atm · l2/mol2 and b = 0.0171 l/mol.)

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:00
An object is located 30.0 cm from a concave mirror. the focal length is 15.0 c,. what is the image distance? a. 30.0 cm b. -10 cm c. 10.0 cm d. -30.0 cm
Answers: 1

Physics, 22.06.2019 06:30
From 0 to 5 seconds john pushed a box 5 meters. from 5 to 10 seconds, paul pushed the same box another 5 meters. who did more work? a. john b. paul c. john and paul did the same amount of work.
Answers: 1

Physics, 22.06.2019 07:20
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 22.06.2019 19:30
Coal contains energy. a. light b. kinetic c. chemical d. mechanical
Answers: 1
You know the right answer?
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the...
Questions


English, 05.07.2019 23:00


Mathematics, 05.07.2019 23:00

History, 05.07.2019 23:00

History, 05.07.2019 23:00

Spanish, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00


Mathematics, 05.07.2019 23:00


Mathematics, 05.07.2019 23:00

Mathematics, 05.07.2019 23:00

Health, 05.07.2019 23:00



History, 05.07.2019 23:00