Physics, 20.12.2019 19:31 unicornpoop54
An 80.0-g piece of copper, initially at 295°c, is dropped into 250 g of water contained in a 300-g aluminum calorimeter; the water and calorimeter are initially at 10.0°c.
what is the final temperature of the system? (specific heats of copper and aluminum are 0.092 0 and 0.215 cal/g⋅°c, respectively. cw = 1.00 cal/g°c)
a. 12.8°c
b. 16.5°c
c. 28.4°c
d. 32.1°c

Answers: 2


Another question on Physics

Physics, 22.06.2019 12:50
Match each vocabulary term to its definition. 1. electrons neutral subatomic particles found in the nucleus of the atom 2. neutron lowest energy position of an electron in an atom 3. photon the path of an electron around the nucleus of an atom 4. ground state negatively charged, subatomic particles 5. protons packet of energy of specific size 6. element substance with only one type of atom 7. orbital positively charged, subatomic particles found in the nucleus of the atom
Answers: 1

Physics, 22.06.2019 12:50
The combining of light nuclei is called blank. blank as in not actually blank. you know what im tryin to say.
Answers: 1

Physics, 22.06.2019 21:00
The first law of thermodynamics states that heat added to a system is neither created nor destroyed but is as it changes into other forms of energy.
Answers: 1

You know the right answer?
An 80.0-g piece of copper, initially at 295°c, is dropped into 250 g of water contained in a 300-g a...
Questions

English, 20.01.2021 23:20

English, 20.01.2021 23:20



Mathematics, 20.01.2021 23:20


Mathematics, 20.01.2021 23:20

Mathematics, 20.01.2021 23:20

Arts, 20.01.2021 23:20


Mathematics, 20.01.2021 23:20


Mathematics, 20.01.2021 23:20



History, 20.01.2021 23:20

Arts, 20.01.2021 23:20

Mathematics, 20.01.2021 23:20

English, 20.01.2021 23:20

Mathematics, 20.01.2021 23:20

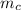 = mass of piece of copper = 80 g
= mass of piece of copper = 80 g 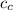 = specific heat of piece of copper = 0.0920 cal/g°C
= specific heat of piece of copper = 0.0920 cal/g°C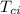 = Initial temperature of piece of copper = 295 °C
= Initial temperature of piece of copper = 295 °C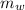 = mass of water = 250 g
= mass of water = 250 g 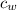 = specific heat of water = 1 cal/g°C
= specific heat of water = 1 cal/g°C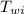 = Initial temperature of piece of copper = 10 °C
= Initial temperature of piece of copper = 10 °C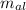 = mass of calorimeter = 300
= mass of calorimeter = 300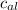 = specific heat of calorimeter = 0.215 cal/g°C
= specific heat of calorimeter = 0.215 cal/g°C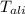 = Initial temperature of calorimeter = 10 °C
= Initial temperature of calorimeter = 10 °C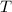 = Final equilibrium temperature
= Final equilibrium temperature