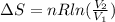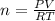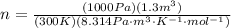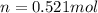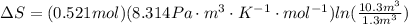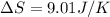Physics, 23.12.2019 18:31 lavelma2011
Amonatomic ideal gas in equilibrium at a pressure of 3 kpa and a temperature of 300 k is initially confined in a volume of 1.3 m^3 by a wall. the wall is suddenly removed and a new equilibrium state with the volume 10.4 m^3 is reached. you may assume that the system is isolated, such that there is no exchange of heat or work with its surrounding environment.1. what is the total change in entropy? a. δs = 9.01 j/k b. δs = 31.5 j/k c. δs = 0 j/k d. δs = 1.1 j/k e. δs = 63.1 j/k

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:00
Will you chech and finish these for me, because i am stumped with them.
Answers: 1

Physics, 22.06.2019 08:00
If a substance is in the gas phase, which of qualities of the gas will stay constant? a: volume b: mass c: shape d: position of particles
Answers: 2


Physics, 22.06.2019 11:00
Which of the following are guidelines to follow for obtaining accurate observations
Answers: 2
You know the right answer?
Amonatomic ideal gas in equilibrium at a pressure of 3 kpa and a temperature of 300 k is initially c...
Questions







History, 03.09.2020 23:01

Chemistry, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

Engineering, 03.09.2020 23:01





Mathematics, 03.09.2020 23:01


English, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

Advanced Placement (AP), 03.09.2020 23:01