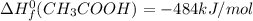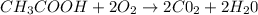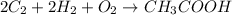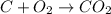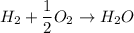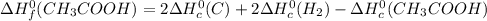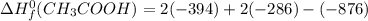Physics, 24.12.2019 23:31 robert7248
Calculate the standard enthalpy of formation of ethanoic acid given that the standard enthalpy of combustion for carbon is –394 kj mol-1 , hydrogen is –286 kj mol-1 and ethanoic acid is –876 kj mol-1

Answers: 3


Another question on Physics

Physics, 22.06.2019 10:30
Carbon is allowed to diffuse through a steel plate 15 mm thick. the concentrations of carbon at the two faces are 0.65 and 0.30 kg c/m^3 fe, which are maintained constant. if the preexponential and activation energy are 6.2 x 10-7 m2 /s and 80,000 j/mol, respectively, compute the temperature at which the diffusion flux is 1.43 x 10^-9 kg/m^2 -s.
Answers: 3

Physics, 22.06.2019 15:30
Ineed ! using proper grammar, spelling, and punctuation, write at least one 5 sentence paragraph describing 3 ways we use the elements of the electromagnetic spectrum (ems) in our everyday lives.
Answers: 1

Physics, 22.06.2019 21:00
Earth's atmosphere is composed of mostly two gases. name the two gases that make up the majority of the atmosphere and use the pie chart to tell the percentages of each.
Answers: 2

Physics, 22.06.2019 21:10
For the below questions, consider a consumer that consumes two goods, x and z with the following utility function. u with bar on top space equals space x to the power of 1 third end exponent z to the power of 2 over 3 end exponent suppose initial values for income and the prices of goods x and z are y equals 90, p subscript x equals space 10, and p subscript z equals 15 respectively, then the price of good x falls to syntax error from line 1 column 89 to line 1 column 100. unexpected '\'.. what is the magnitude of the total effect
Answers: 3
You know the right answer?
Calculate the standard enthalpy of formation of ethanoic acid given that the standard enthalpy of co...
Questions

Physics, 11.07.2019 22:30









History, 11.07.2019 22:30


Computers and Technology, 11.07.2019 22:30

Mathematics, 11.07.2019 22:30



History, 11.07.2019 22:30