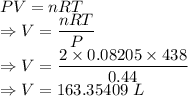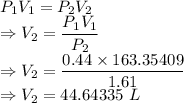Physics, 25.12.2019 17:31 live4dramaoy0yf9
Two moles of helium gas initially at 438 k and 0.44 atm are compressed isothermally to 1.61 atm. find the final volume of the gas. assume that helium behaves as an ideal gas. the universal gas constant is 8.31451 j/k · mol.

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:20
Calculate the capacitance of a system that stores 2.0 x 10^-10c of charge at 100.0 v. use c=q/v. a. 2.0 x 10^-12 f b. 2.0 x 10^-8 f c. 5.0 x 10^11 f d. 5.0 x 10^7 f
Answers: 1

Physics, 22.06.2019 07:00
Within a pendulum, as potential energy decreases, energy increases. a. heat b. kinetic c. frictional d. gravitational
Answers: 1

Physics, 22.06.2019 15:30
1kg of water needs to recieve 4200j of energy to go from 90 degrees to 91 degrees. what is the specific heat capacity of water? what are the units?
Answers: 1

Physics, 23.06.2019 02:50
Aweather balloon has a volume of 52.5 liters at a temperature of 252 k. the balloon is released and rises to an altitude where the temperature is 295 k. how does this temperature change affect the gas particle motion?
Answers: 1
You know the right answer?
Two moles of helium gas initially at 438 k and 0.44 atm are compressed isothermally to 1.61 atm. fin...
Questions


Computers and Technology, 29.02.2020 01:27






Mathematics, 29.02.2020 01:27