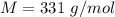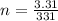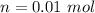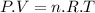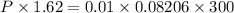Physics, 27.12.2019 05:31 salvadorperez26
A3.31 g sample of lead nitrate, pb(no 3 ) 2 , molar mass = 331 g/mol, is heated in an evacuated cylinder with a volume of 1.62 l. the salt decomposes when heated according to the equation: . assuming complete decomposition, what is the pressure in the cylinder after decomposition and cooling to a temperature of 300 k? assume the pbo takes up negligible volume.

Answers: 2


Another question on Physics

Physics, 22.06.2019 14:40
The experiment done in lab is repeated, using a ball that has unknown mass m. you plot your data in the form of f 2 versus m/l, with f in rev/s, m in kg, and l in m. your data falls close to a straight line that has slope 3.19 m/(kg · s2). use g = 9.80 m/s2 and calculate the mass m of the ball.
Answers: 1

Physics, 22.06.2019 15:50
The california mussel (mytilus californianus) attaches itself to a rock or other solid surface with a bundle of filaments known as the byssus. imagine that 15.0 j of work is done to stretch the distal end of the byssus. it releases 10.8 j of thermal energy as it relaxes. what is the resilience of the distal end of the byssus?
Answers: 2

Physics, 22.06.2019 16:00
What is friction? how does it affect the motion of an object?
Answers: 1

Physics, 22.06.2019 18:30
Which word expression describes how much to calculate pressure
Answers: 1
You know the right answer?
A3.31 g sample of lead nitrate, pb(no 3 ) 2 , molar mass = 331 g/mol, is heated in an evacuated cyli...
Questions


Mathematics, 26.04.2021 21:40


Mathematics, 26.04.2021 21:40


Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40


Business, 26.04.2021 21:40


Mathematics, 26.04.2021 21:40


Arts, 26.04.2021 21:40

Social Studies, 26.04.2021 21:40



Mathematics, 26.04.2021 21:40


Biology, 26.04.2021 21:40

Physics, 26.04.2021 21:40

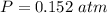
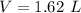 The mass of the sample,
The mass of the sample, 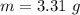 The temperature,
The temperature, 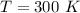 The molar mass of lead nitrate is
The molar mass of lead nitrate is