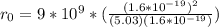The binding energy of a potassium chloride molecule (k0) is 4.43 ev. the ionization energy of a potassium atom is 4.3 ev, and the electron affinity of chlorine is 3.6 ev. use these data to estimate the equilibrium separation between the two atomsin the kci molecule. explain why your result is only an estimate and not a precise value.

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:00
How does the amount of energy required to hold each proton and neutron in the nucleus compare to the energy released when they are removed?
Answers: 3

Physics, 22.06.2019 02:30
The boy of mass 30 kg is at r1= 2 meters from the fulcrum. of the girl is 45 kg, at what r2 must she sit so that they are balanced ?
Answers: 3


You know the right answer?
The binding energy of a potassium chloride molecule (k0) is 4.43 ev. the ionization energy of a pota...
Questions





Computers and Technology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Chemistry, 20.09.2020 16:01




Mathematics, 20.09.2020 16:01

History, 20.09.2020 16:01

Business, 20.09.2020 16:01

Computers and Technology, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

English, 20.09.2020 16:01



Mathematics, 20.09.2020 16:01


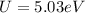

 is the charge on one body,
is the charge on one body,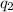 is the charge on the other body,
is the charge on the other body, is the electric constant or permittivity of free space or permittivity of the vacuum
is the electric constant or permittivity of free space or permittivity of the vacuum