
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 149.8 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:20
Which quantities are scalars? choose all that apply a.distance b.speed c. acceleration d.velocity
Answers: 2

Physics, 22.06.2019 09:00
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 22.06.2019 18:30
Blood pressure the total amount of blood the heart pumps in one minute 2. cardiac output the number of times your heart beats in a minute 3. dilate the amount of blood that the heart can pump in a single beat 4. heart rate the force exerted on the walls of the blood vessels by the blood that moves through them 5. stroke volume to widen or get larger in size
Answers: 3

Physics, 22.06.2019 18:30
In which situation is the acceleration of a car negative? question 5 options: a)the velocity of a car was 75 km/hr over 4 hours. b)the velocity of a car reduced from 50 km/hr over one minute. c)the velocity of a car increased from 40 km.h to 75 km/h over 15 minutes. d)the velocity of a car was 45 km/hr at 2: 00pm and at 4: 00pm its velocity was 85 km/ht.
Answers: 2
You know the right answer?
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions








Social Studies, 27.08.2019 05:10



Computers and Technology, 27.08.2019 05:10


History, 27.08.2019 05:10







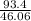 = 2.02 moles
= 2.02 moles

