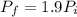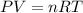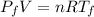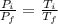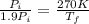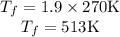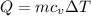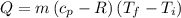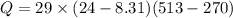Physics, 18.01.2020 00:31 RickyGotFanz4867
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas is increased until the final pressure equals 1.9 times the initial pressure. the heat capacity at constant pressure of the gas is what is the heat absorbed by the gas? let the ideal-gas constant r = 8.314 j/(mol • k).
7.0 kj
170 kj
230 kj
110 kj
-52 kj

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:20
Alfred pushes on a heavy box, but cannot move it. the box has a lot of inertia motion friction gravity
Answers: 1

Physics, 22.06.2019 09:40
Aturntable a is built into a stage for use in a theatrical production. it is observed during a rehearsal that a trunk b starts to slide on the turntable 10 s after the turntable begins to rotate. knowing that the trunk undergoes a constant tangential acceleration of 0.31 m/s2, determine the coefficient of static friction between the trunk and the turntable.
Answers: 3

Physics, 22.06.2019 11:30
Acaterpillar tries to climb straight up a wall a meter high, but for every 2 cm up it climbs, it slides down 1 cm. eventually, it reaches the top. when it reaches the top, it does not pull itself over so it will slide down 1 cm. what is the total displacement traveled?
Answers: 3

Physics, 22.06.2019 12:30
An ice-making machine inside a refrigerator operates in a carnot cycle. it takes heat from liquid water at 0.0 degrees celsius and rejects heat to a room at a temperature of 19.2 degrees celsius. suppose that liquid water with a mass of 76.3kg at 0.0 degrees celsius is converted to ice at the same temperature. take the heat of fusion for water to be l_f = 3.34*10^5 j/kg.how much energy e must be supplied to the device? express your answer in joules.
Answers: 1
You know the right answer?
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas...
Questions




English, 12.06.2020 04:57

Mathematics, 12.06.2020 04:57


History, 12.06.2020 04:57

Health, 12.06.2020 04:57

History, 12.06.2020 04:57

English, 12.06.2020 04:57






History, 12.06.2020 04:57

Chemistry, 12.06.2020 04:57



Mathematics, 12.06.2020 04:57

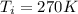
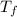 , the initial pressure will be
, the initial pressure will be 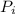 and the final pressure will be,
and the final pressure will be,