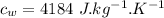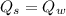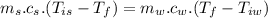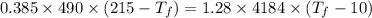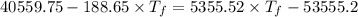Physics, 23.01.2020 00:31 simplydimps22owbohb
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 "c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steamproduced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’taffect the outcome. as we’ll see in the next section.)

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:30
Work out sian speed for the first 30 minutes of her journey. give your answer in km/h.
Answers: 1

Physics, 22.06.2019 05:00
Which statements describe the movement of ocean currents around the globe? check all that apply. strong winds force warm water to sink to the ocean floor. the coriolis effect causes warm and cold water to mix. cool dense water sinks to the ocean floor. warm water replaces cool surface water. wind blowing parallel to the shore causes upwelling of cool water.
Answers: 1

Physics, 22.06.2019 12:30
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1

You know the right answer?
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimete...
Questions


Chemistry, 05.04.2020 17:19

Computers and Technology, 05.04.2020 17:19


History, 05.04.2020 17:20

Mathematics, 05.04.2020 17:20

Mathematics, 05.04.2020 17:21


Mathematics, 05.04.2020 17:21



Arts, 05.04.2020 17:22


Mathematics, 05.04.2020 17:23

Social Studies, 05.04.2020 17:23

Biology, 05.04.2020 17:23

Geography, 05.04.2020 17:23

English, 05.04.2020 17:24


Health, 05.04.2020 17:24

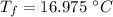
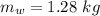 initial temperature of water,
initial temperature of water, 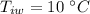 mass of steel,
mass of steel, 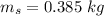 initial temperature of steel,
initial temperature of steel, 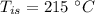 specific heat capacity of steel,
specific heat capacity of steel, 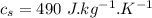 specific heat capacity of water,
specific heat capacity of water,