
Physics, 29.01.2020 14:40 bronkosarecool
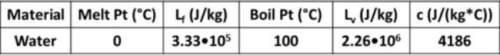
A0.0500 kg ice cube starts at -18.5°c. how much heat does it take to melt it completely? (don't forget, you have to warm it up to the melting point first.) (unit=j)

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
An automobile steering wheel is shown. what is the ideal mechanical advantage? if the ama is 8, what is the efficiency of the steering wheel?
Answers: 1

Physics, 22.06.2019 09:40
Aturntable a is built into a stage for use in a theatrical production. it is observed during a rehearsal that a trunk b starts to slide on the turntable 10 s after the turntable begins to rotate. knowing that the trunk undergoes a constant tangential acceleration of 0.31 m/s2, determine the coefficient of static friction between the trunk and the turntable.
Answers: 3

Physics, 22.06.2019 16:40
An owl dives toward the ground with a constant velocity of 4.40 m/s at 53.0° below the horizontal. the sun is directly overhead and casts a shadow of the owl directly below it. what is the speed (in m/s) of its shadow on level ground?
Answers: 3

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3
You know the right answer?
A0.0500 kg ice cube starts at -18.5°c. how much heat does it take to melt it completely? (don't for...
Questions




Mathematics, 19.09.2019 06:01




Mathematics, 19.09.2019 06:01

Social Studies, 19.09.2019 06:01

Health, 19.09.2019 06:01



History, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01



Mathematics, 19.09.2019 06:01

Chemistry, 19.09.2019 06:01



