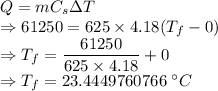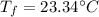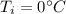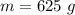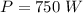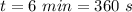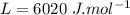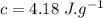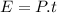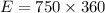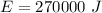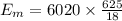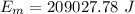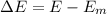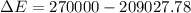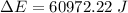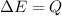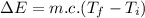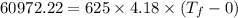Physics, 11.02.2020 05:32 mariaaalopezz
Ice cubes at 0°C with a total mass of 625 g are put in a microwave oven and heated with 750. W (750. J/s) of energy for 6.00 minutes. What is the final temperature of the water from the melted ice? Assume all of the microwave energy is absorbed by the ice/water and no heat loss by the ice/water. The enthalpy of fusion for ice is 6.02 kJ/mol and the heat capacity for water is 4.18 J/g・°C.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:10
What do elements in a family tend to share. a.) similar periods b.) similar groups c.) similar atomic symbols d.) similar chemical properties and characteristics
Answers: 2


Physics, 22.06.2019 13:50
Observations show that interstellar clouds can have almost any shape and
Answers: 1

Physics, 22.06.2019 17:00
(a) if the pressure in gas is doubled while its volume is held constant, by what factor do (i) vrms and (ii) change? (b) is it possible to boil water at room temperature (20oc) without heating it? explain.
Answers: 3
You know the right answer?
Ice cubes at 0°C with a total mass of 625 g are put in a microwave oven and heated with 750. W (750....
Questions

Mathematics, 18.02.2021 02:00


Biology, 18.02.2021 02:00

Health, 18.02.2021 02:00



Mathematics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00


Physics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00



Mathematics, 18.02.2021 02:00

Health, 18.02.2021 02:00

Mathematics, 18.02.2021 02:00



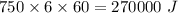
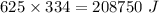
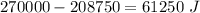
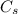 = Heat capacity for water = 4.18 J/g・°C.
= Heat capacity for water = 4.18 J/g・°C.