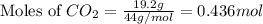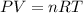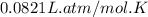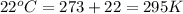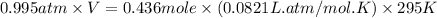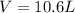Physics, 11.02.2020 23:31 kiarabermudez754
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus. Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constant temperature of 22 degrees C. Assume that the initial volume of dry ice is negligible and that CO2 behaves like an ideal gas.

Answers: 2


Another question on Physics

Physics, 22.06.2019 10:30
Astone weighing 1.5 kilograms is resting on a rock at a height of 20 meters above the ground. the stone rolls down 10 meters and comes to rest on a patch of moss. the gravitational potential energy of the stone on the moss is joules.
Answers: 1

Physics, 22.06.2019 16:00
Apersons beliefs and general outlook, which act like filters on the information they receive are called ?
Answers: 1

Physics, 22.06.2019 17:40
The weights of bags filled by a machine are normally distributed with a standard deviation of 0.05 kilograms and a mean that can be set by the operator. at what level should the mean weight be set if it required that only 1% of the bags weigh less than 9.5 kilograms? round the answer to 2 decimal places.
Answers: 1

Physics, 22.06.2019 18:30
Which form of cell division creates the sperm and egg, resulting in half of the chromosomes as the other cells?
Answers: 1
You know the right answer?
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus...
Questions


Mathematics, 01.09.2019 11:30





Mathematics, 01.09.2019 11:30

Mathematics, 01.09.2019 11:30




Geography, 01.09.2019 11:30

Biology, 01.09.2019 11:30

Biology, 01.09.2019 11:30

Mathematics, 01.09.2019 11:30

Mathematics, 01.09.2019 11:30



History, 01.09.2019 11:30

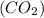 .
.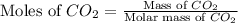
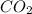 = 44 g/mole
= 44 g/mole