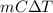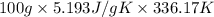Physics, 12.02.2020 02:01 loishsu4936
A mass of 0.1 kg of helium fills a 0.2 m3 rigid tank at 350 kPa. The vessel is heated until the pressure is 700 kPa. Calculate the a) the temperature change of helium [deg. C], and b) the total amount of heat required for this process [kJ].

Answers: 3


Another question on Physics

Physics, 22.06.2019 06:30
Air initially at 0.75 bar, 1000 k, and occupying a volume of 0.12 m^3 undergoes two processes. process 1-2: the air is compressed isothermally until the volume is halved. process 2-3: the air undergoes a constant pressure process until the volume is halved again. assume ideal gas behavior. a) determine the mass of the air, in kg. b) the work and the heat transfer for each of the two processes, in kj. (100 kj = 1 bar . m^3)
Answers: 1



Physics, 23.06.2019 12:30
Acar's position in relation to time is plotted on the graph. what is the car's average velocity for segment c? a) -4 m/s b) -0.25 m/s c) 0.25 m/s d) 4 m/s
Answers: 1
You know the right answer?
A mass of 0.1 kg of helium fills a 0.2 m3 rigid tank at 350 kPa. The vessel is heated until the pres...
Questions

Chemistry, 11.07.2019 00:00






Mathematics, 11.07.2019 00:00


History, 11.07.2019 00:00

History, 11.07.2019 00:00

Mathematics, 11.07.2019 00:00

Mathematics, 11.07.2019 00:00

English, 11.07.2019 00:00

Mathematics, 11.07.2019 00:00






Chemistry, 11.07.2019 00:00

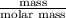
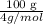
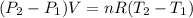
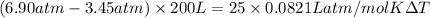
 = 336.17 K
= 336.17 K