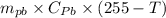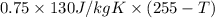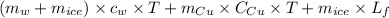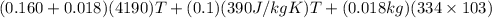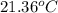A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal equilibrium at atmospheric pressure.
Part A
If 0.750kg of lead at a temperature of 255 c is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:10
Atotal charge of –6.50 µc is uniformly distributed within a sphere that has a radius of 0.150 m. what is the magnitude and direction of the electric field at 0.300 m from the surface of the sphere? a) 2.89 × 105 n/c, radially inward b) 6.49 × 105 n/c, radially outward c) 4.69 × 105 n/c, radially inward d) 9.38 × 105 n/c, radially outward e) 1.30 × 106 n/c, radially inward
Answers: 3

Physics, 22.06.2019 21:00
If the specific heat of a metal is 0.850 j/g °c, what is its atomic weight?
Answers: 2

Physics, 22.06.2019 22:30
Use the illustration to describe how the electromagnetic spectrum changes as its frequency moves from radio waves to higher energy gamma waves in terms of wavelength and amplitude.
Answers: 1

Physics, 23.06.2019 03:50
For most stars, what does a higher temperature tend to to with? a lower luminosity a higher luminositya red color none of the above
Answers: 1
You know the right answer?
A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal e...
Questions

Mathematics, 10.02.2020 03:39

Social Studies, 10.02.2020 03:39

Biology, 10.02.2020 03:39




Biology, 10.02.2020 03:40


English, 10.02.2020 03:40


History, 10.02.2020 03:40





Mathematics, 10.02.2020 03:42


Mathematics, 10.02.2020 03:43

Social Studies, 10.02.2020 03:43

English, 10.02.2020 03:43

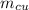 ) = 0.1 kg
) = 0.1 kg
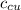 ) = 390 J/kg K
) = 390 J/kg K
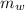 ) = 0.160 kg
) = 0.160 kg
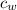 ) = 4190 J/kg K
) = 4190 J/kg K
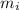 ) = 0.018 kg
) = 0.018 kg
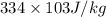
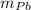 ) = 0.75 kg
) = 0.75 kg
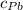 ) = 130 J/kg K
) = 130 J/kg K