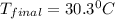An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C, in an insulated container. Part A Assuming that no heat is lost to the surroundings, what is the temperature of the entire water sample after all of the ice has melted?

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:20
An object thrown vertically upward from the surface of a celestial body at a velocity of 36 m/s reaches a height of sequalsminus0.9tsquaredplus36t meters in t seconds. a. determine the velocity v of the object after t seconds. b. when does the object reach its highest point? c. what is the height of the object at the highest point? d. when does the object strike the ground? e. with what velocity does the object strike the ground? f. on what intervals is the speed increasing?
Answers: 1

Physics, 22.06.2019 08:40
The system is released from rest with the cable taut, and the homogeneous cylinder does not slip on the rough incline. determine the angular acceleration of the cylinder and the minimum coeffi cient s of friction for which the cylinder will not slip.
Answers: 2

Physics, 22.06.2019 13:40
An ideal otto cycle has a compression ratio of 10.5, takes in air at 90 kpa and 40°c, and is repeated 2500 times per minute. using constant specific heats at room temperature, determine the thermal efficiency of this cycle and the rate of heat input if the cycle is to produce 90 kw of power.
Answers: 2

Physics, 22.06.2019 21:00
Which of the the following are examples of projectile motion of both projectile motion and two dimensional motion
Answers: 3
You know the right answer?
An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C,...
Questions


Mathematics, 18.12.2020 01:20

Mathematics, 18.12.2020 01:20


Mathematics, 18.12.2020 01:20

Chemistry, 18.12.2020 01:20

Mathematics, 18.12.2020 01:20

Engineering, 18.12.2020 01:20

Mathematics, 18.12.2020 01:20

History, 18.12.2020 01:20


Chemistry, 18.12.2020 01:20

Mathematics, 18.12.2020 01:20

Mathematics, 18.12.2020 01:20






Advanced Placement (AP), 18.12.2020 01:20


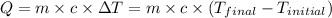 .......(1)
.......(1)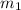 = mass of ice = 21.5 g
= mass of ice = 21.5 g = mass of water = 500.0 g
= mass of water = 500.0 g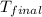 = final temperature = ?
= final temperature = ?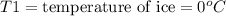
 = temperature of water =
= temperature of water =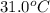
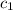 = specific heat of ice=
= specific heat of ice= 
 = specific heat of water =
= specific heat of water = 
![21.5\times 2.1\times (T_{final}-0)=-[500.0\times 4.184\times (T_{final}-31.0)]](/tpl/images/0513/4270/003f3.png)