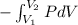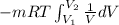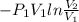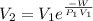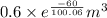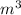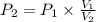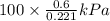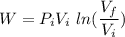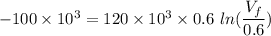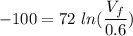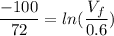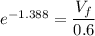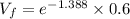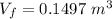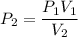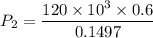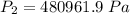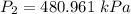An ideal gas contained in a piston–cylinder device undergoes an isothermal compression process which begins with an initial pressure and volume of 120 kPa and 0.6 m3, respectively. During the process, there is a heat transfer of 60 kJ from the ideal gas to the surroundings. Determine the volume and the pressure at the end of the process. The properties of air are R = 0.287 kJ/kg·K and cv = 0.718 kJ/kg·K.

Answers: 2


Another question on Physics


Physics, 22.06.2019 06:30
Which features on mars point to the possibility of liquid water on the planet? impact craters with sharp rims volcanic cones with craters gullies and stream-like channels mountain ranges with faults
Answers: 1

Physics, 22.06.2019 08:00
If a balloon is taken outside on a very cold day, what will occur?
Answers: 1

Physics, 22.06.2019 12:30
Consider a 1000 w iron whose base plate is made of 0.5 cm thick aluminum alloy 2024-t6 (ρ = 2770 kg/m3 and cp = 875 j/kg°c). the base plate has a surface area of 0.03 m2. initially, the iron is in thermal equilibrium with the ambient air at 22°c. assuming 90% of the heat generated in the resistance wires is transferred to the plate, determine the minimum time needed for the plate temperature to reach 200°c.
Answers: 1
You know the right answer?
An ideal gas contained in a piston–cylinder device undergoes an isothermal compression process which...
Questions


Mathematics, 01.07.2019 20:00

Social Studies, 01.07.2019 20:00

Biology, 01.07.2019 20:00

Mathematics, 01.07.2019 20:00

World Languages, 01.07.2019 20:00

Mathematics, 01.07.2019 20:00

Biology, 01.07.2019 20:00

Mathematics, 01.07.2019 20:00

Physics, 01.07.2019 20:00




Social Studies, 01.07.2019 20:00


Mathematics, 01.07.2019 20:00

Mathematics, 01.07.2019 20:00

Social Studies, 01.07.2019 20:00

Social Studies, 01.07.2019 20:00