Physics, 20.02.2020 20:50 jonathanvega424
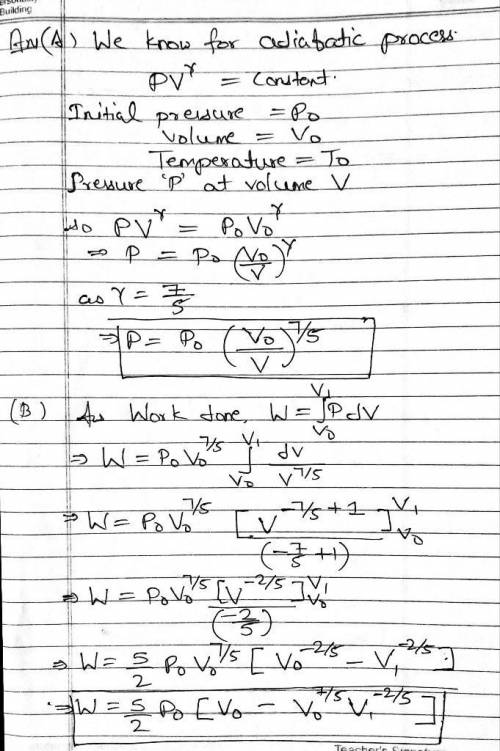
In this problem you are to consider an adiabaticexpansion of an ideal diatomic gas, which means that the gas expands with no addition or subtraction of heat.
Assume that the gas is initially at pressure p0, volume V0, and temperature T0. In addition, assume that the temperature of the gas is such that you can neglect vibrational degrees of freedom. Thus, the ratio of heat capacities is γ=Cp/CV=7/5.
Note that, unless explicitly stated, the variable γ should not appear in your answers--if needed use the fact that γ=7/5 for an ideal diatomic gas.
Part A
Find an analytic expression for p(V), the pressure as a function of volume, during the adiabatic expansion.
Express the pressure in terms of V and any or all of the given initial values p0, T0, and V0.
Correct
p(V) =
p0(V0V)75
Part B
At the end of the adiabatic expansion, the gas fills a new volume V1, where V1>V0. Find W, the work done by the gas on the container during the expansion.
Express the work in terms of p0, V0, and V1. Your answer should not depend on temperature.
Part C
Find ΔU, the change of internal energy of the gas during the adiabatic expansion from volume V0 to volume V1.
Express the change of internal energy in terms of p0, V0, and/or V1.
Need Part B and C

Answers: 3


Another question on Physics

Physics, 22.06.2019 21:30
The diagram shows a boulder rolling down a hill into a valley and then up the opposite hill. at which position does the boulder have the greatest kinetic energy? ab c d
Answers: 2

Physics, 22.06.2019 21:30
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created
Answers: 3

Physics, 22.06.2019 23:50
What is part of a line has one endpoint and continues in one direction?
Answers: 1

Physics, 23.06.2019 00:30
What is the coldest temperature ever recorded in san antonio?
Answers: 1
You know the right answer?
In this problem you are to consider an adiabaticexpansion of an ideal diatomic gas, which means that...
Questions





Chemistry, 20.05.2021 04:40



Mathematics, 20.05.2021 04:40


History, 20.05.2021 04:40



History, 20.05.2021 04:40


Mathematics, 20.05.2021 04:40

History, 20.05.2021 04:40


Mathematics, 20.05.2021 04:40

Mathematics, 20.05.2021 04:40

Biology, 20.05.2021 04:40

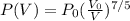
![W= \frac{5}{2} P_{0}[V_{0}-V_{0}^{7/5}V_{1}^{-2/5}]](/tpl/images/0517/8982/53b5c.png)
![\delta U = \frac{5}{2}P_{0}[V_{0}^{7/5}V_{1}^{-2/5}-V_{0}]](/tpl/images/0517/8982/2dece.png)