Physics, 21.02.2020 23:05 ciralove2004
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K and a pressure of 10 atm. At this temperature, KC 0.25(mol/dm3)2. Calculate the equilibrium conversion for each of the following situations:

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:30
If you quadruple the temperature of a black body, by what factor will the total energy radiated per second per square meter increase? 1,024 64 16 4 256 submit answer
Answers: 1

Physics, 22.06.2019 05:40
Two polarizers are oriented at 40∘ to each other and plane-polarized light is incident on them. if only 16 percent of the light gets through both of them, what was the initial polarization direction of the incident light?
Answers: 2

Physics, 22.06.2019 07:30
Gas cloud 1 is likely to form a star. gas cloud 2 is not. based on this information, match the given conditions with each cloud
Answers: 2

Physics, 22.06.2019 15:50
The california mussel (mytilus californianus) attaches itself to a rock or other solid surface with a bundle of filaments known as the byssus. imagine that 15.0 j of work is done to stretch the distal end of the byssus. it releases 10.8 j of thermal energy as it relaxes. what is the resilience of the distal end of the byssus?
Answers: 2
You know the right answer?
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K a...
Questions

English, 26.12.2021 23:50


Computers and Technology, 27.12.2021 01:00




Mathematics, 27.12.2021 01:00

Health, 27.12.2021 01:00



Mathematics, 27.12.2021 01:00

English, 27.12.2021 01:00

English, 27.12.2021 01:00

Mathematics, 27.12.2021 01:00


English, 27.12.2021 01:00

Medicine, 27.12.2021 01:00

History, 27.12.2021 01:00


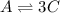

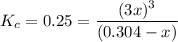
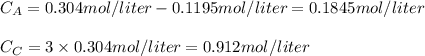
![\% = [0.304mol/liter-0.1845mol/liter]/(0.304mol/liter)\times 100](/tpl/images/0519/7722/78e3b.png)