

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:00
Fill in the blank. the number of each type of element in the compound (other than 1) is represented by a small number to the right of the element symbol.
Answers: 1

Physics, 22.06.2019 00:00
Name three different units of energy used to measure heat and describe what type of situations each is usually used.
Answers: 2

Physics, 22.06.2019 06:20
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

Physics, 22.06.2019 21:10
For the below questions, consider a consumer that consumes two goods, x and z with the following utility function. u with bar on top space equals space x to the power of 1 third end exponent z to the power of 2 over 3 end exponent suppose initial values for income and the prices of goods x and z are y equals 90, p subscript x equals space 10, and p subscript z equals 15 respectively, then the price of good x falls to syntax error from line 1 column 89 to line 1 column 100. unexpected '\'.. what is the magnitude of the total effect
Answers: 3
You know the right answer?
Calculate the change in internal energy (ΔE) for a system that is giving off 25.0 kJ of heat and is...
Questions





Computers and Technology, 30.07.2019 12:00

Social Studies, 30.07.2019 12:00


Computers and Technology, 30.07.2019 12:00

Mathematics, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

Biology, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

History, 30.07.2019 12:00


Biology, 30.07.2019 12:00

Mathematics, 30.07.2019 12:00


Computers and Technology, 30.07.2019 12:00

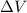 = (12 - 6) L = 6 L
= (12 - 6) L = 6 L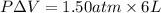
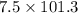
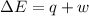
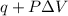
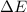 ) for a system is 24.240 kJ.
) for a system is 24.240 kJ.

