Physics, 26.02.2020 03:54 1341220857
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The quartz sample starts off a insulated 95.8 °C and the temperature of the water starts off at 15.0 °C, when the temperature of the water stops changing it's 17.7 °C. The pressure remains constant at 1 atm.
Calculate the mass of the quartz sample.

Answers: 3


Another question on Physics

Physics, 21.06.2019 15:00
The equation that describes a transverse wave on a string is y = (0.0120 m)sin[(927 rad/s)t - (3.00 rad/m)x] where y is the displacement of a string particle and x is the position of the particle on the string. the wave is traveling in the +x direction. what is the speed v of the wave?
Answers: 1

Physics, 22.06.2019 23:00
1700 j of energy is lost from 0.14 kg object , the temperature decreases from 50°c to 45°c what is the specific heat of this object, amd what is the material ?
Answers: 1

Physics, 22.06.2019 23:30
Aswimming pool whose volume is 10 comma 000 gal contains water that is 0.01% chlorine. starting at tequals0, city water containing 0.003% chlorine is pumped into the pool at a rate of 5 gal/min. the pool water flows out at the same rate. what is the percentage of chlorine in the pool after 1 hour? when will the pool water be 0.006% chlorine?
Answers: 1

Physics, 22.06.2019 23:50
The star nearest to our sun is proxima centauri, at a distance of 4.3 light-years from the sun. how far away, in km, is proxima centauri from the sun?
Answers: 3
You know the right answer?
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see...
Questions


History, 08.12.2020 01:00


Biology, 08.12.2020 01:00

Chemistry, 08.12.2020 01:00





English, 08.12.2020 01:00

Physics, 08.12.2020 01:00







History, 08.12.2020 01:00


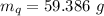
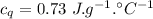
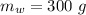
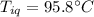
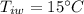
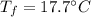
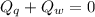
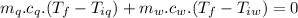
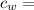 specific heat of water =
specific heat of water = 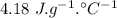 ,
,