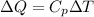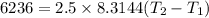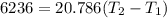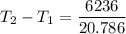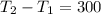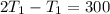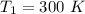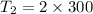Physics, 27.02.2020 02:06 donterriuscollier
2. One mole of a monatomic ideal gas undergoes a reversible expansion at constant pressure, during which the entropy of the gas increases by 14.41 J/K and the gas absorbs 6236 J of thermal energy. Calculate the initial and final temperatures of the gas.

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:30
Boxing gloves are padded to lessen the force of a blow. (a) calculate the force exerted by a boxing glove on an opponent’s face, if the glove and face compress 7.50 cm during a blow in which the 7.00-kg arm and glove are brought to rest from an initial speed of 10.0 m/s. (b) calculate the force exerted by an identical blow in the gory old days when no gloves were used and the knuckles and face would compress only 2.00 cm. (c) discuss the magnitude of the force with glove on. does it seem high enough to cause damage even though it is lower than the force with no glove?
Answers: 1

Physics, 22.06.2019 22:10
M1 = 2.8 kg, m2 = 6.72 kg, m3 = 11.2 kg, byas in .is ,is .toof m3 it 0.91 m. (in m/s)
Answers: 3


Physics, 23.06.2019 06:30
Acopper sheet has a volume of 100 cm³. if the density of copper is 8.9 g/cm³, what is the mass of the sheet? a. 11 g b. 8.9 g c. 189 g d. 890 g
Answers: 1
You know the right answer?
2. One mole of a monatomic ideal gas undergoes a reversible expansion at constant pressure, during w...
Questions






Mathematics, 19.06.2021 19:10

Physics, 19.06.2021 19:10


English, 19.06.2021 19:10

World Languages, 19.06.2021 19:10


Mathematics, 19.06.2021 19:10

Mathematics, 19.06.2021 19:10

English, 19.06.2021 19:10

Computers and Technology, 19.06.2021 19:20


Mathematics, 19.06.2021 19:20

Mathematics, 19.06.2021 19:20

Mathematics, 19.06.2021 19:20

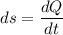
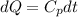
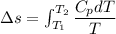
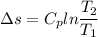
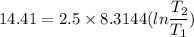
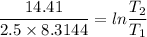
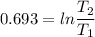
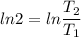
 ...(I)
...(I)