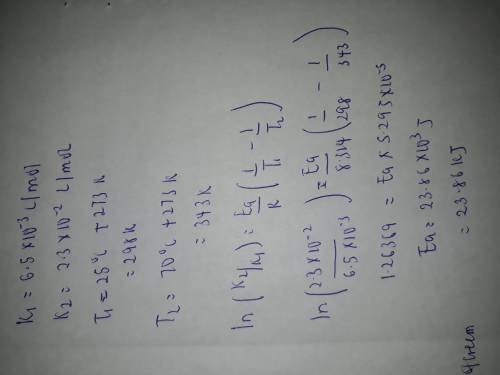Physics, 27.02.2020 03:19 Samonerob2002
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-3L/mol and at 70 oC it was found to be 2.30 x 10-2 L/mol. Calculate the activation energy of this reaction.

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:40
What happens when chlorine reacts with bromine? a. electrons move from the chlorine atoms to the bromine atoms. b. electrons move from the bromine atoms to the chlorine atoms. c. electrons are shared between the chlorine atoms and the bromine atoms. d. electrons become delocalized among the atoms.
Answers: 2


Physics, 22.06.2019 15:00
What happens when a rubber rod is rubbed with a piece of fur?
Answers: 1

Physics, 22.06.2019 18:00
Aprisoner is forced to go into one of three rooms, but he can choose which room. the first room is ablaze with fire. the second one is rigged with explosives that will go off as soon as he enters. the third contains a pair of lions who haven't eaten in years. which room should he choose to survive?
Answers: 2
You know the right answer?
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-...
Questions



History, 19.12.2020 20:20


History, 19.12.2020 20:20



Mathematics, 19.12.2020 20:20



Computers and Technology, 19.12.2020 20:20




Mathematics, 19.12.2020 20:20

Health, 19.12.2020 20:20


Mathematics, 19.12.2020 20:20