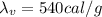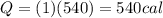Physics, 01.03.2020 21:26 lilybrok04
A. How many calories are needed to raise the temperature of 1 gram of water by 1 °C?
B. How many calories are needed to melt 1 gram of ice at 0 °C?
C. How many calories are needed to vaporize 1 gram of boiling water at 100 °C?

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:20
Describe the path of the electric current through a circuit?
Answers: 1

Physics, 21.06.2019 22:30
Ann walks 80 meters on a straight line 33 ∘ north of east starting at point 1. draw ann's path. represent ann's walk with a vector of length 80 meters. draw the vector starting at point 1. the length given in the display is in meters.
Answers: 1

Physics, 21.06.2019 23:30
1.in one challenge on the titan games, competitors have to lift 200 pounds up a long ramp. angel is able to move the weight in 42 seconds. anthony gets it done in only 38 seconds. which statement is true? angel has more power than anthony. angel does more work than anthony. anthony does more work than angel. anthony has more power than angel. 2.a mountain climber exerts 41,000 j of work to climb a cliff. how much power does the climber need if she wants to finish in only 500 seconds? power = work / time 20,500,000 watts 82 watts 0.0122 watts 41,500 watts 3.your family is moving to a new apartment. while lifting a box 83 joules of work is done to put the box on a truck, you exert an upward force of 75 n for 3 s. how much power is required to do this? (hint: you only need two of the 3 numbers given! ) power = work / time 249 watts 25 watts 2075 watts 27.7 watts
Answers: 1

Physics, 22.06.2019 05:30
Acombination reaction is when two or more combine to form one product. a decomposition reaction is when a substance breaks down into two or more simpler substances in a chemical reaction.
Answers: 1
You know the right answer?
A. How many calories are needed to raise the temperature of 1 gram of water by 1 °C?
B. H...
B. H...
Questions




English, 18.10.2019 03:30

Mathematics, 18.10.2019 03:30



Mathematics, 18.10.2019 03:30

History, 18.10.2019 03:30

English, 18.10.2019 03:30


Mathematics, 18.10.2019 03:30



Mathematics, 18.10.2019 03:30





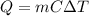
 is the change in temperature
is the change in temperature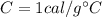 is the specific heat capacity of water
is the specific heat capacity of water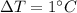 is the change in temperature
is the change in temperature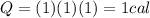
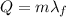
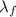 is the specific latent heat of fusion of the substance
is the specific latent heat of fusion of the substance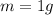
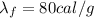
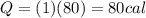
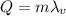
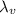 is the specific latent heat of vaporization of the substance
is the specific latent heat of vaporization of the substance