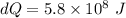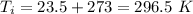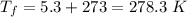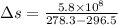Physics, 03.03.2020 00:51 curlyheadnikii
On a hot summer day, 3.50 ✕ 106 J of heat transfer into a parked car takes place, increasing its temperature from 36.5°C to 44.4°C. What is the increase in entropy (in J/K) of the car due to this heat transfer alone?
On a winter day, a certain house loses 5.80 ✕ 108 J of heat to the outside (about 550,000 Btu). What is the total change in entropy (in J/K) due to this heat transfer alone, assuming an average indoor temperature of 23.5°C and an average outdoor temperature of 5.30°C?

Answers: 3


Another question on Physics

Physics, 22.06.2019 11:30
A100-watt light bulb illuminates a solar cell. the electricity from the solar cell operates a water pump that delivers 1 watt of power. what is the efficiency of the system?
Answers: 2

Physics, 22.06.2019 16:00
The electric field direction is defined by the direction of the force felt by (select one of the following answers): 1. a negative charge. 2. a positive charge. 3. both positive and negative charges.
Answers: 2


Physics, 22.06.2019 21:00
During a car accident, a 125kg driver is moving at 31m/s and in 1.5s is brought to rest by an inflating air bag. what is the magnitude of the change in momentum to the driver
Answers: 2
You know the right answer?
On a hot summer day, 3.50 ✕ 106 J of heat transfer into a parked car takes place, increasing its tem...
Questions



Mathematics, 11.09.2021 05:30



Health, 11.09.2021 05:30




Mathematics, 11.09.2021 05:30

Mathematics, 11.09.2021 05:40





Mathematics, 11.09.2021 05:40


Business, 11.09.2021 05:40


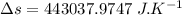
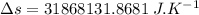
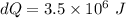
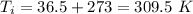
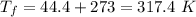
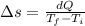
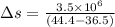 (heat is transferred into the system of car)
(heat is transferred into the system of car)