Physics, 06.03.2020 01:03 mayaholmes
A certain monatomic gas inside a cylinder is at a temperature of 22°C. It takes 353 J of work done on the gas to compress it and increase the temperature to 145°C. If there are originally 8.2 moles of gas inside the cylinder, calculate the quantity of heat flowing into or out of the gas. (Indicate the direction with the sign of your answer. Let "into the gas" be positive, and "out of the gas" be negative.)

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:00
Will you chech and finish these for me, because i am stumped with them.
Answers: 1


Physics, 22.06.2019 06:50
What is the stall speed at sea level of an airplane that weights 10000 lbs., has a wing area of 300ft^2, and a maximum lift coefficient of 1.4? what is the stall speed if flaps that double cl,max are applied?
Answers: 1

Physics, 22.06.2019 12:10
Ablock having mass m slides down an inclined plane. the force of friction between the block and the inclined plane is f, the block's weight is m g, and the normal force is n. (a) draw a free – body force diagram showing the forces acting on the block. (b) write down all relevant newton’s equations for a given situation.
Answers: 1
You know the right answer?
A certain monatomic gas inside a cylinder is at a temperature of 22°C. It takes 353 J of work done o...
Questions


English, 30.08.2019 21:50





Mathematics, 30.08.2019 21:50


Mathematics, 30.08.2019 21:50

Biology, 30.08.2019 21:50



History, 30.08.2019 21:50


English, 30.08.2019 21:50

Physics, 30.08.2019 21:50

Health, 30.08.2019 21:50

Mathematics, 30.08.2019 21:50


Mathematics, 30.08.2019 21:50




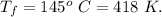

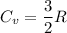 Here R is universal gas constant
Here R is universal gas constant  )
)