
Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same type of gas, but container B has twice the number of particles and twice the volume of container A. The average kinetic energy per molecule in container B is :
A. Twice that for container A
B. The same as that for container A
C. Half that for container A
D. Impossible to determine

Answers: 2


Another question on Physics

Physics, 22.06.2019 03:00
Which of the following is not a part of the respiratory system? a. pharynx b. trachea c. pancreas d. larynx hurrim timed
Answers: 1

Physics, 22.06.2019 03:30
Scout feels ill one day before school. her mother puts a thermometer in her mouth and the temperature begins to climb to 100°f. what is happening between the cooler thermometer and scout's body? a) scout's body is applying a force to the particles in the thermometer. b) kinetic energy is being transferred from her mouth to the thermometer. c) potential energy is being lost by the thermometer and gained by scout's body. d) the potential energy stored in foods is converted to mechanical energy to raise the mercury in the thermometer.
Answers: 1

Physics, 22.06.2019 08:00
Why is it important always to use horizontal bars in unit fractions when performing unit conversions?
Answers: 3

Physics, 22.06.2019 09:30
Astone is dropped from a cliff and falls 9.44 meters. what is the speed of the stone when it reaches the ground? a. 13.6 m/sec b. 1.39 m/sec c. 185 m/sec d. 9.80 m/sec
Answers: 3
You know the right answer?
Two containers hold an ideal gas at the same temperature and pressure. Both containers hold the same...
Questions


English, 16.04.2022 20:20



Mathematics, 16.04.2022 20:30



Business, 16.04.2022 21:10





English, 16.04.2022 21:30



Mathematics, 16.04.2022 21:40


Geography, 16.04.2022 21:50


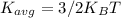
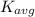 is the average kinetic energy per molecule and
is the average kinetic energy per molecule and 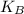 is the Boltzmann constant
is the Boltzmann constant 

