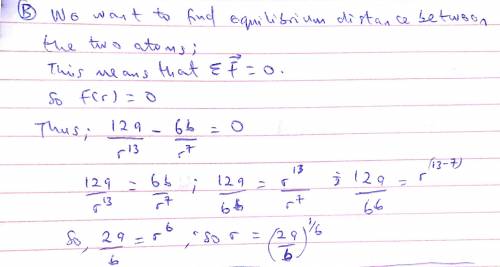Physics, 07.03.2020 06:12 Pumpkinputters
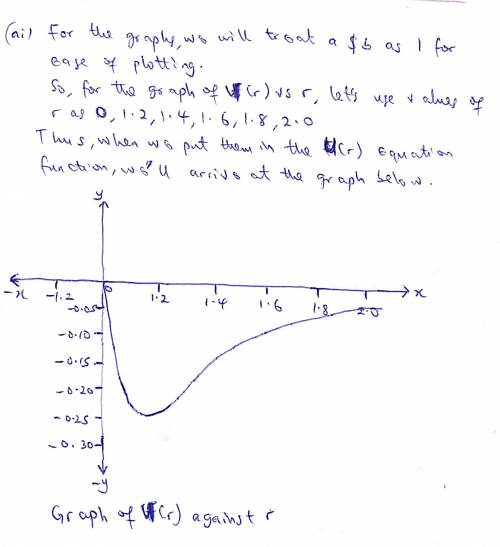
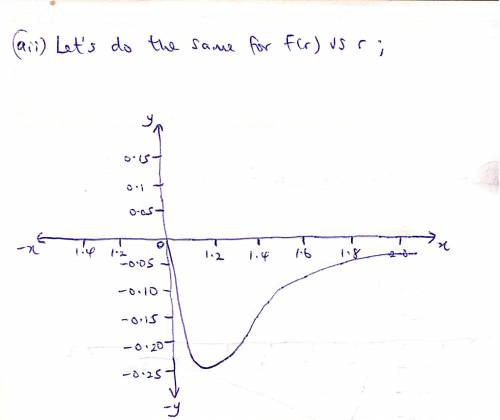
The potential energy of two atoms in a diatomic molecule is approximated by U(r)=(a/r12)−(b/r6), where r is the spacing between atoms and a and b are positive constants. (a) Find the force F(r) on one atom as a function of r. Draw two graphs: one of U(r) versus r and one of F(r) versus r. (b) Find the equilibrium distance between the two atoms. Is this equilibrium stable? (c) Suppose the distance between the two atoms is equal to the equilibrium distance found in part (b). What minimum energy must be added to the molecule to dissociate it—that is, to separate the two atoms to an infinite distance apart? This is called the dissociation energy of the molecule. (d) For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13×10−10m and the dissociation energy is 1.54×10−18J per molecule. Find the values of the constants a and b.

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:00
In which of the following cases is work being done on an object? question 2 options: pushing against a locked door carrying a box down a corridor pulling a trailer up a hill suspending a heavy weight with a strong chain
Answers: 2

Physics, 22.06.2019 06:40
Use the right-hand rule for magnetic force to determine the charge on the moving particle. this is a charge.
Answers: 1

Physics, 22.06.2019 07:30
Examine the nuclear reacti why is this classified as a nuclear reaction rather than a chemical reaction? it is not balanced. a new compound is formed. a change has occurred in a nucleus. a new element has been formed.
Answers: 2

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1
You know the right answer?
The potential energy of two atoms in a diatomic molecule is approximated by U(r)=(a/r12)−(b/r6), whe...
Questions

Mathematics, 25.11.2021 05:50


Computers and Technology, 25.11.2021 05:50

Mathematics, 25.11.2021 05:50



Mathematics, 25.11.2021 05:50







Physics, 25.11.2021 05:50




Computers and Technology, 25.11.2021 05:50