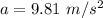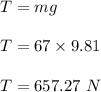Physics, 09.03.2020 20:43 dontcareanyonemo
A gymnast of mass 67.0 kg hangs from a vertical rope attached to the ceiling. You can ignore the weight of the rope and assume that the rope does not stretch. Use the value 9.81m/s2 for the acceleration of gravity.
Calculate in Newtons:
a. The tension T in the rope if the gymnast hangs motionless on the rope.
b. The tension T in the rope if the gymnast climbs the rope at a constant rate.

Answers: 1


Another question on Physics

Physics, 22.06.2019 16:00
Amichelson interferometer operating at a 600 nm wavelength has a 3.02-cm-long glass cell in one arm. to begin, the air is pumped out of the cell and mirror m2 is adjusted to produce a bright spot at the center of the interference pattern. then a valve is opened and air is slowly admitted into the cell. the index of refraction of air at 1.00 atm pressure is 1.00028.how many bright-dark-bright fringe shifts are observed as the cell fills with air?
Answers: 1

Physics, 22.06.2019 16:50
Consider the growth of a 20-nm-diameter silicon nanowire onto a silicon wafer. the temperature of the wafer surface is maintained at 2400 k. assume the thermal conductivity of the silicon nanowire is 20 wm-1k-1 and all its surfaces including the tip are subjected to convection heat transfer with the coefficient h = 1×105 wm-2k-1 and t∞ = 8000 k. when the nanowire grows to l = 300 nm, what is the temperature of the nanowire tip (t (x =
Answers: 1

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 22.06.2019 19:20
Two kilograms of air within a piston–cylinder assembly executes a carnot power cycle with maximum and minimum temperatures of 800 k and 300 k, respectively. the heat transfer to the air during the isothermal expansion is 60 kj. at the end of the isothermal expansion the volume is 0.4 m3. assume the ideal gas model for the air. determine the thermal efficiency, the volume at the beginning of the isothermal expansion, in m3, and the work during the adiabatic expansion, in kj.
Answers: 1
You know the right answer?
A gymnast of mass 67.0 kg hangs from a vertical rope attached to the ceiling. You can ignore the wei...
Questions



World Languages, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20



English, 26.10.2020 22:20

Computers and Technology, 26.10.2020 22:20

History, 26.10.2020 22:20


History, 26.10.2020 22:20




Chemistry, 26.10.2020 22:20


Mathematics, 26.10.2020 22:20


Biology, 26.10.2020 22:20