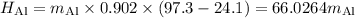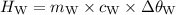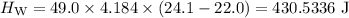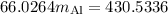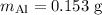A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing 49.0g of water at an initial temperature of 22.0°C. The two were allowed to come to thermal equilibrium and the final temperature was 24.1°C. The specific heats of water and aluminum are 4.184 J/g °C and 0.902 J/g °C, respectively. What was the mass of the Al piece that was added?

Answers: 3


Another question on Physics

Physics, 22.06.2019 22:50
The illuminance of a surface varies inversely with the square of its distance from the light source. if the illuminance of a surface is 120 lumens per square meter when its distance from a certain light source is 6 meters, by how many meters should the distance of the surface from the source be increased to reduce its illuminance to 30 lumens per square meter?
Answers: 3

Physics, 23.06.2019 00:20
Amaterial that provides resistance to the flow of electric current is called a(an) (1 points)
Answers: 1

Physics, 23.06.2019 09:30
What is the volume of 2400kg of gasoline (petrol) if the density of petrol is 0.7g/km3
Answers: 1

Physics, 23.06.2019 21:00
The portion of the visible spectrum that appears brightest to the human eye is approximately 560 nm in wavelength, which corresponds to yellow-green. what is the frequency of this light?
Answers: 1
You know the right answer?
A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing...
Questions

Business, 31.07.2019 10:00




Biology, 31.07.2019 10:00

Social Studies, 31.07.2019 10:00

English, 31.07.2019 10:00

Mathematics, 31.07.2019 10:00

Health, 31.07.2019 10:00



History, 31.07.2019 10:00

Advanced Placement (AP), 31.07.2019 10:00

Arts, 31.07.2019 10:00

Mathematics, 31.07.2019 10:00


History, 31.07.2019 10:00



Health, 31.07.2019 10:00