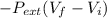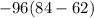A mixture of nitrogen and argon gas is expanded from a volume of to a volume of 62 L to 84 L, while the pressure is held constant at 96 atm . Calculate the work done on the gas mixture. Round your answer to significant digits, and be sure it has the correct sign (positive or negative).

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:50
The intensity of a polarized electromagnetic wave is 12 w/m^2. part a) what will be the intensity after passing through a polarizing filter whose axis makes the angle θ=0∘ with the plane of polarization? part b) what will be the intensity after passing through a polarizing filter whose axis makes the angle θ=30∘ with the plane of polarization? part c) what will be the intensity after passing through a polarizing filter whose axis makes the angle θ=45∘ with the plane of polarization? part d) what will be the intensity after passing through a polarizing filter whose axis makes the angle θ=60∘ with the plane of polarization? part e) what will be the intensity after passing through a polarizing filter whose axis makes the angle θ=90∘ with the plane of polarization?
Answers: 1

Physics, 22.06.2019 05:30
Which of the following are considered noble gases? a. bromine b. neon c. argon d. chlorine
Answers: 1

Physics, 22.06.2019 08:00
You have a pick-up truck that weighed 4,000 pounds when it was new. you are modifying it to increase its ground clearance. when you are finished
Answers: 1

Physics, 22.06.2019 13:30
Select the three ways that the parallel-plate capacitor differs from a car battery.
Answers: 1
You know the right answer?
A mixture of nitrogen and argon gas is expanded from a volume of to a volume of 62 L to 84 L, while...
Questions

Spanish, 14.04.2020 20:03



History, 14.04.2020 20:03


Mathematics, 14.04.2020 20:03

Law, 14.04.2020 20:03

Mathematics, 14.04.2020 20:03


Mathematics, 14.04.2020 20:03


Mathematics, 14.04.2020 20:03

History, 14.04.2020 20:03



History, 14.04.2020 20:03

Biology, 14.04.2020 20:03



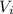 = 62 L
= 62 L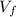 = 84 L
= 84 L