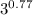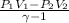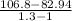Physics, 12.03.2020 06:16 moonlighthowl4537
Carbon dioxide gas in a piston–cylinder assembly expands from an initial state where p1 60 lbf/in.2 , V1 1.78 ft3 to a final pressure of p2 20 lbf/in.2 The relationship between pressure and volume during the process is pV1.3 constant. For the gas, calculate the work done, in Convert your answer to Btu.

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz. what wavelength is produced?
Answers: 2

Physics, 22.06.2019 07:30
Carbon-14 is a radioactive element that undergoes beta decay. which force is responsible for allowing carbon-14 to become stable? electromagnetic gravitational weak nuclear strong nuclear
Answers: 2

Physics, 22.06.2019 09:30
Suppose you increase your walking speed from 7 m/s to 13 m/s in a period of 2 s. what is your acceleration?
Answers: 2

Physics, 22.06.2019 12:30
An engine undergoes a cycle as shown in the diagram. during a complete cycle the work done by the gas is 28.5×103 j and the energy transferred thermally out is 99.4×103 j. there are 1.5×1024 gas molecules and they have three active degrees of freedom. 6. (6 pts) during which process(es) is energy transferred thermally into the gas? a. 1→2 b. 2→3 c. 4→1 d. 1→2 and 4→1 e. none of the above
Answers: 3
You know the right answer?
Carbon dioxide gas in a piston–cylinder assembly expands from an initial state where p1 60 lbf/in.2...
Questions




Computers and Technology, 09.04.2021 04:10





Computers and Technology, 09.04.2021 04:10



Computers and Technology, 09.04.2021 04:10

Mathematics, 09.04.2021 04:10

Social Studies, 09.04.2021 04:10

Physics, 09.04.2021 04:10


Mathematics, 09.04.2021 04:10

Mathematics, 09.04.2021 04:10


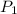 ) = 60
) = 60 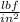
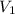 )= 1.78
)= 1.78 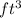
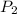 ) = 20
) = 20 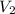 ) = We have to calculate.
) = We have to calculate.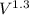
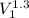 =
= 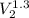
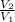 =
= ![[\frac{P_{1} }{P_{2}}]^{0.77} }](/tpl/images/0544/6982/f8f9e.png)